Biomedical 3D printing firm ArcomedLab has reportedly established the world’s largest case list of 3D printed craniomaxillofacial implants.
Since 2018, the company has completed 700 successful cases throughout Latin America, including in Chile, Peru, Colombia, and Mexico. 3D printed in a polyetheretherketone (PEEK) biopolymer, the company’s cranial implants are personalized to meet each patient’s specific needs.
Possessing biocompatibility and resistance to bodily fluids, PEEK is a leading choice for the production of medical devices. The material features mechanical properties similar to human bone, is stable across various temperatures, and offers good radiolucency.
The 3D printed implants can also store and deliver liquid drugs where they are needed through ArcomedLab’s patented gravity-induced drip mechanism. This technology enables drugs to be delivered up to 20 days after surgery.
“For us it is an honor to lead the biomedical 3D printing sector in the region, with a case list of patients that makes us extremely proud and with PEEK and Titanium 3D printing technology that is unique in Latin America,” commented ArcomedLab’s CEO mad Co-Founder Ilan Rosenberg.
Having achieved this milestone, the company has also announced the appointment of Daniel Martínez as its new Innovation Strategy and Growth Manager.
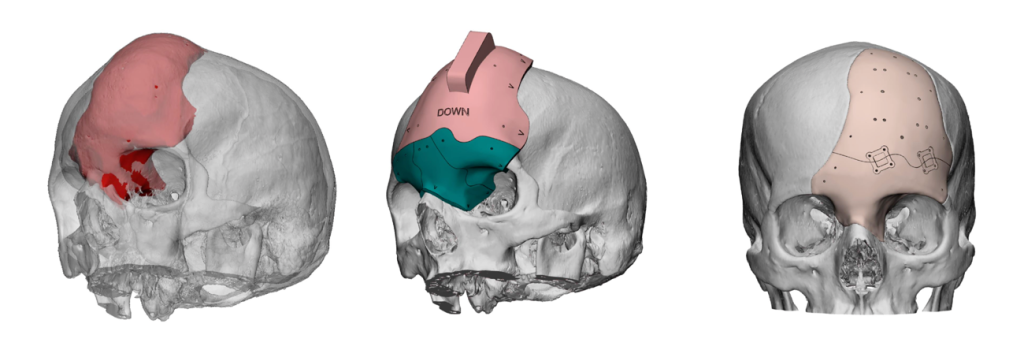
ArcomedLab’s 3D printed implants
ArcomedLab produces its personalized 3D printed cranial implants at its facility in Chile. This features a dedicated clean room for 3D printing personalized implants and medical devices in a controlled and sterile environment.
In addition to its substantial case-list, the company has also broken the record for the world’s largest 3D printed cranial implant. Designed for a child with a partially formed skull, the 3D printed implant almost covers the entire head from the supraorbital ridges to the occipital bone
Leveraging FDM and SLA technology, ArcomedLab is not limited to 3D printing cranial components. It also produces a range of custom-made chin, zygoma, angle and orbit implants.

The company now hopes to expand its operations further with the help of its new Innovation Strategy and Growth Manager. Martínez brings a wealth of experience to his new role, having previously worked in the MedTech, innovation, and clinical sectors. A professor at Adolfo Ibáñez University in Santiago, he co-founded material development firm Copper3D in 2016 and served as its Chief Innovation Officer until February 2024.
Copper3D specializes in the development of 3D printable antimicrobial nanocomposites for the medical device, animal care, food and consumer products industries. In 2020, Martínez led the company in the development of a 3D printed breastfeeding device. The product was designed to act as an interface between mother and child, inactivating HIV found in breast milk.
ArcomedLab hopes that Martínez’s insights and strategic vision will help to drive its growth strategy in the Latin America and US markets.
Martínez stated that he is “excited to join ArcomedLab and contribute to advancing biomedical 3D printing in the orthopedic area, on upper and lower extremities as well as spine personalized solutions.”
Looking to the future, the company plans to expand its presence by creating new partnerships with leading clinical groups and universities throughout Latin America.
“Our second goal is to leverage the ArcomedLab Institute’s resources and know-how to further develop innovative academic programs in the field of biomedical 3D printing in alliance with top universities and medical schools, to accelerate the introduction of these technologies in clinical settings and improve patient outcomes with cost-effective and personalized solutions,” added Martínez.
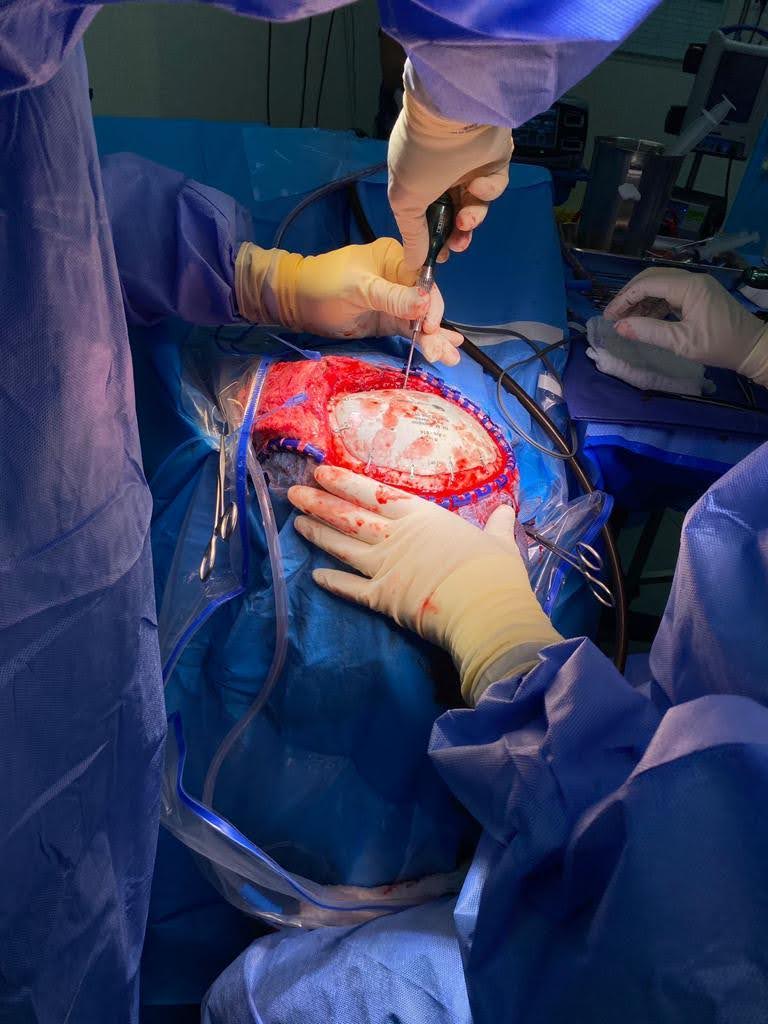
Is 3D printing the future for cranial implants?
The medical sector is witnessing increasing adoption of 3D printing. This adoption is especially prevalent in the production of cranial implants. A February 2023 report from Acumen Research and Consulting estimated that the cranial implants market was valued at approximately $1.2 billion in 2021.
According to 3D printer manufacturer 3D Systems, this market could reach nearly $2.1 billion by 2030. To tap into this growing industry, the company secured FDA 510K clearance for its patient-specific, VSP PEEK 3D printed cranial implants.
This comprehensive workflow combines 3D modeling software with 3D Systems’ EXT 220 MED 3D printer, and Evonik VESTAKEEP i4 3DF PEEK material within a predefined production process. According to 3D Systems, this additive manufacturing approach can create patient-specific cranial implants with 85% less material than traditional machining. Last year, the company used its point-of-care medical 3D printing technology to create a patient-specific 3D printed cranial implant for a procedure at the University Hospital Basel.
Elsewhere, Researchers from the Graz University of Technology (TU Graz) and the Medical University of Graz in Austria, created an automated design software for 3D printable cranial implants. The program uses deep learning algorithms to automatically restore the missing part of a skull. It then generates an STL file for a patient-specific implant, which can then be 3D printed on-site at the medical facility. This drastically reduces lead times, and can also reduce the discomfort of patients by optimizing the implant’s fit.
Want to help select the winners of the 2024 3D Printing Industry Awards? Join the Expert Committee today.
What does the future of 3D printing hold?
What near-term 3D printing trends have been highlighted by industry experts?
Subscribe to the 3D Printing Industry newsletter to keep up to date with the latest 3D printing news.
You can also follow us on Twitter, like our Facebook page, and subscribe to the 3D Printing Industry Youtube channel to access more exclusive content.
Featured image shows digital models of 3D printed cranial implants. Image via ArcomedLab.

